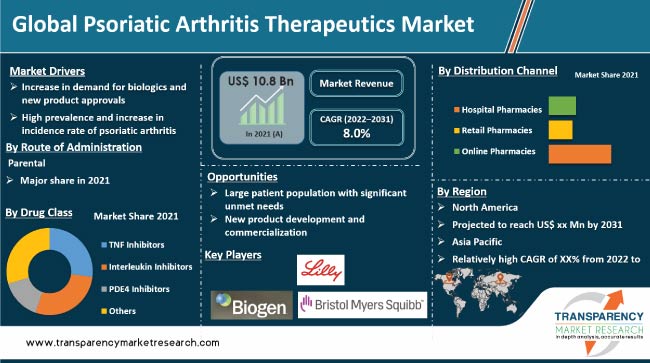
Transparency Market Research (TMR) has published a new report titled, “Psoriatic Arthritis Therapeutics Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026”. According to the report, the global psoriatic arthritis therapeutics market was valued at US$ 6,011.7 Mn in 2017. It is projected to expand at a CAGR of 11.4% from 2018 to 2026. High prevalence of psoriatic arthritis, large unmet medical needs, and new product approvals are anticipated to drive the global market in the next few years. North America is expected to dominate the global psoriatic arthritis therapeutics market during the forecast period, followed by Europe. Potential unmet medical needs for moderate to severe psoriatic arthritis, increase in adoption of biologic drugs, and approvals of new therapeutic drug classes in the U.S. and Europe are likely to drive the psoriatic arthritis therapeutics market in these regions during the forecast period. The market in Asia Pacific is projected to expand at a high CAGR during the forecast period.
High prevalence and increase in incidence rate of psoriatic arthritis to drive market
High prevalence and rise in incidence rate of psoriatic arthritis across the globe is a key factor driving the global psoriatic arthritis therapeutics market. Chances of developing psoriatic arthritis among patients with psoriasis is high, and the number of patients with psoriatic arthritis has been increasing significantly in the last few years. According to the International Federation of Psoriasis Association, more than 125 million people were affected with psoriasis, globally, in 2015. It is estimated that between 10% and 30% of patients with psoriasis tend to develop psoriatic arthritis. According to the study published in the Journal of the American Academy of Dermatology, in 2013, Denmark had the highest prevalence rate of psoriatic arthritis, with around 42% prevalence rate, while the lowest prevalence rates were recorded in Canada and Belgium. Thus, the high prevalence and substantial rise in incidence rate of psoriasis and psoriasis-led psoriatic arthritis across globe is a key factor driving the global psoriatic arthritis therapeutics market.
Interleukin inhibitors and PDE4 inhibitors drugs to propel the psoriatic arthritis therapeutics market
The report offers a detailed segmentation of the global psoriatic arthritis therapeutics market based on different therapeutic drug classes approved and commercialized for psoriatic arthritis. Based on drug class, the global market has been segmented into TNF inhibitors, interleukin inhibitors, PDE4 inhibitors, and others. Interleukin inhibitors drugs have evolved as new therapeutic drug class for the treatment of psoriatic arthritis when other therapeutic drugs classes, especially TNF alpha inhibitors, fail to relieve the symptoms of psoriatic arthritis. Interleukin inhibitor drugs, especially IL-17A inhibitor drugs, have shown the highest clinical efficacy, as compared to any other biologic drug available, to relive the symptoms of psoriatic arthritis. Interleukin inhibitors have been recently approved for the treatment of psoriatic arthritis and are increasingly gaining popularity among physicians across the globe. Currently, only four interleukin inhibitors ? Cosentyx (Secukinumab), Stelara (USTEKINUMAB), Tremfya (Guselkumab) and Taltz (Ixekizumab) ? have been approved for the treatment of psoriatic arthritis. Interleukin inhibitors drug sales are estimated to rise about four times by the end of 2026. PDE4 inhibitor is the new therapeutic drug that was approved by the USFDA, in March 2014, for the treatment of psoriatic arthritis. Currently, Otezla (Apremilast) is the only approved PDE4 inhibitor drug for psoriatic arthritis treatment. Within one year of launch, Otezla generated a sale of more than US$ 1 Billion in the year 2016.
For More Details, Request A PDF Brochure Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=61917
Parenteral route of administration to dominate global market
In terms of route of administration, the global psoriatic arthritis therapeutics market has been segmented into oral route, parenteral route, and topical routes. The parenteral route segment dominated the global market in 2017. It is likely to maintain its dominance by the end of 2026. Majority of the therapeutic drugs available for the treatment of moderate to severe psoriatic arthritis are biologics drugs that are administered through the subcutaneous and intravenous routes. The high cost and large volume prescription of biologic drugs contributed to the leading share held by the parenteral route segment in 2017.
Hospital pharmacies segment to account for high market share
In terms of distribution channel, the global psoriatic arthritis therapeutics market has been segregated into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment is projected to account for a significant share of the market by 2026. Rise in the number of hospital admissions of patients with psoriatic arthritis and high cost of treatment are anticipated to contribute to the high share of the segment by the end of 2026. Promising biologic product pipeline of leading biopharmaceutical companies is likely to receive approval and commercialization in the near future. Biologic drugs are most commonly and easily available at hospital pharmacy stores. Hence, expected launch and commercialization of biologic therapeutic drugs is likely to drive the hospital pharmacies segment during the forecast period.
Asia Pacific offers high incremental opportunity
The psoriatic arthritis therapeutics market in Asia Pacific is projected to expand at a high CAGR during the forecast period. High prevalence of psoriatic arthritis in highly populated countries, such as China and India, rapidly improvement in health care infrastructure, increase in access to health care facilities, and rise in per capita health care expenditure in the region are likely to fuel the market in Asia Pacific during the forecast period. Large base of pharmaceutical companies in countries such as India, Japan, and China also drives the market in the region. High demand and increased adoption of biologic drugs in developed markets, such as Australia & New Zealand, are projected to propel the psoriatic arthritis therapeutics market in Asia Pacific. For instance, in Australia, the number of patients with severe psoriasis receiving treatment under the Pharmaceutical Benefit Scheme (PBS) with biologics has increased by more than 60% between 2014 and 2016.
Key trend of M&A among leading players to diversify and strengthen psoriatic arthritis product portfolio
The global psoriatic arthritis therapeutics market is highly consolidated, with a few global players accounting for a major share in respective regions. Leading players in psoriatic arthritis therapeutics are engaged in mergers, acquisitions, and strategic collaborations to broaden their psoriatic arthritis therapeutics portfolio. For instance, more than 20 mergers, acquisitions, and strategic collaborations have taken place between 2014 and 2018. Most biopharmaceutical companies have invested significantly in clinical R&D for the development of psoriatic arthritis therapeutics products. Key players operating in the psoriatic arthritis therapeutics market include AbbVie, Inc., Janssen Biotech, Inc., Novartis AG, Amgen, Inc., Celgene Corporation, Pfizer, Inc., Eli Lilly and Company, UCB, Inc., Biogen Inc., and Bristol-Myers and Squibb Company.