The global meningococcal vaccines market was valued at approximately US$ 3.3 Bn in 2017. It is projected to expand at a compound annual growth rate (CAGR) of more than 12.0% from 2018 to 2026, according to a new report published by Transparency Market Research (TMR) titled ‘Meningococcal Vaccines Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026’. Expansion of the health care industry, government initiatives, increase in health care expenditure, rise in product approvals, and increase in patient population are projected to augment the global market from 2018 to 2026. North America and Europe are likely to account for a dominant share of the global meningococcal vaccines market, owing to the presence of key players in these developed regions as well as high rate of adoption of advanced healthcare facilities for meningococcal vaccines in these regions. Development of health care infrastructure and high medical expenditure by governments in emerging economies, such as China and India, are likely to boost the meningococcal vaccines market in Asia Pacific at a CAGR of 13.7% from 2018 to 2026.
Developing countries reshaping global health and development to propel market
Developing countries such as Brazil, Russia, India, China, and South Africa are increasingly engaging in global health improvisation and expansion. However, these countries continue to face health challenges. This has been witnessed by China overtaking Japan, in 2011, to become the second-largest global economy. Brazil and India rank sixth and ninth, respectively. These countries are also investing significantly in health innovation, increasingly influencing health practices across the globe. The Government of India is also increasingly investing in early stage research and development in order to develop innovative health technologies. This has been witnessed by the development of MenAfriVac vaccine, specifically designed for the meningitis belt in Sub-Saharan Africa. Serum Institute of India Ltd., which manufactures MenAfriVac, has played a critical role in reducing the prices and improving access to this vaccine. Hence, production of high quality, low cost vaccines by developing countries is likely to present significant benefits.
Increasing education and awareness through various non-profit organizations
The National Association of School Nurses, a non-profit specialty nursing organization, in collaboration with Sanofi Pasteur, is working in the Voices of Meningitis campaign. It is a public health initiative to help prevent children and teens from getting infected by meningococcal disease. These non-profit organizations, such as the National Meningitis Association (NMA), welcome partnerships by providing opportunities to collaborate with government agencies, corporations, and other non-profit organizations in order to increase awareness about meningococcal disease, its prevention, and the urgent need for immediate treatment following diagnosis. Visits to health care facilities should be used to assess immunization status and administer recommended vaccines in order to provide access to vaccines for as many adolescents as possible. Alternative sites, such as schools or pharmacies, can also be utilized to maximize vaccination opportunities. Increased coverage rates of meningococcal immunization programs worldwide are likely to further increase access to vaccines.
For More Details, Request A PDF Brochure Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1666
Maintenance of cold chains to deliver vaccines leading to high cost of vaccination may hamper market
Maintaining a cold chain (comprising a series of refrigerators and ice-chilled carriers maintaining temperature between 2 degree Celsius and 8 degree Celsius for vaccine storage) is a major challenge while delivering vaccines to the needy, especially in developing countries. Developing such vaccines that do not depend on very narrow range temperatures for storage and the ability to transport vaccine doses outside the cold chain are estimated to lower the cost of vaccination, improve delivery in developing countries, and provide health care workers greater flexibility in reaching people in need. Presently, there is only one meningococcal vaccine, MenAfriVac, which has been approved for use outside the cold chain. The vaccine was known to be safe and effective after up to four days at 40 ?C. In March 2014, Nature, an international weekly journal of science, published an article mentioning the initial results that demonstrate reduced incidence of meningitis due to MenAfriVac vaccination. However, running these studies could be costly and is known to be helpful only in case of polysaccharide vaccines and not conjugate vaccines, which require continual cold storage and any fluctuation in temperature could result in an ineffective and unusable vaccine.
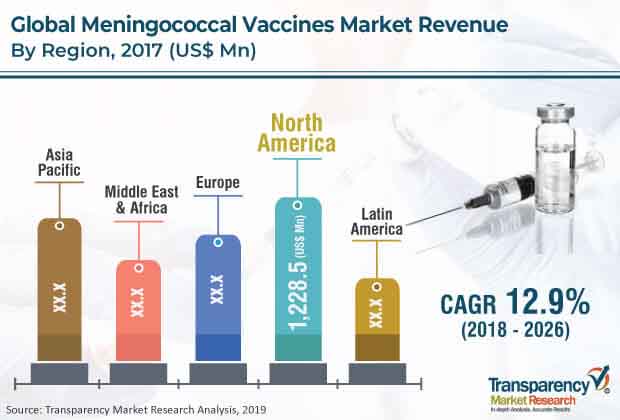
North America leads the market
North America is projected to be a highly lucrative market for meningococcal vaccines during the forecast period. The region held major share of the global meningococcal vaccines market due to high rate of adoption of advanced products and rise in penetration of new entrants with novel technology. The U.S. held a major share of the market in North America as well as globally. The country is a major innovation hub, where the world’s leading companies conduct research and development.
Sanofi S.A., Novartis International, and GSK Plc. to lead market
The report also provides profiles of leading players operating in the global meningococcal vaccines market. They include Sanofi SA, Novartis International, GlaxoSmithKline plc, Pfizer Inc., Nuron Biotech, JN-International Medical Corporation, Serum Institute of India Ltd., Baxter International, and Biomed Pvt. Ltd. Increase in mergers & acquisitions, strategic collaborations, and new product launches are expected to drive the global market during the forecast period. Additionally, strong product portfolio, high brand recognition, and strong geographic presence are expected to boost the market during the forecast period.